
Top Performing Drug – Perjeta (January Edition)
Shots:
-
In continuation of our previous series on the Top-Performing Drug of the month, based on 2021 revenue, this month we have selected Perjeta and prepared a curated analysis report for our readers
-
Perjeta is indicated for the treatment of HER2-positive metastatic breast cancer, neoadjuvant HER2-positive breast cancer, Adjuvant HER2-positive breast cancer
-
PharmaShots presents a concise take on the key features of Perjeta with a detailed analysis of its revenue, clinical trials, alternatives, and approvals. The report is concluded with an engaging SWOT analysis and informative KOL reviews
Active Ingredient: Pertuzumab
Dosage Forms & Strengths: Injection: 420 mg/14 mL single-dose vial
Mechanism of Action: HER2 inhibitor
Originator: Genentech (Roche)
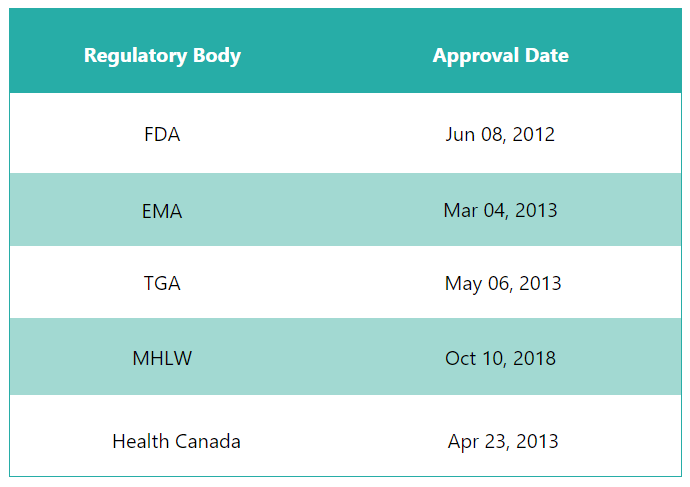
First approvals:
The table below depicts the first approvals of Perjeta by different regulatory agencies.
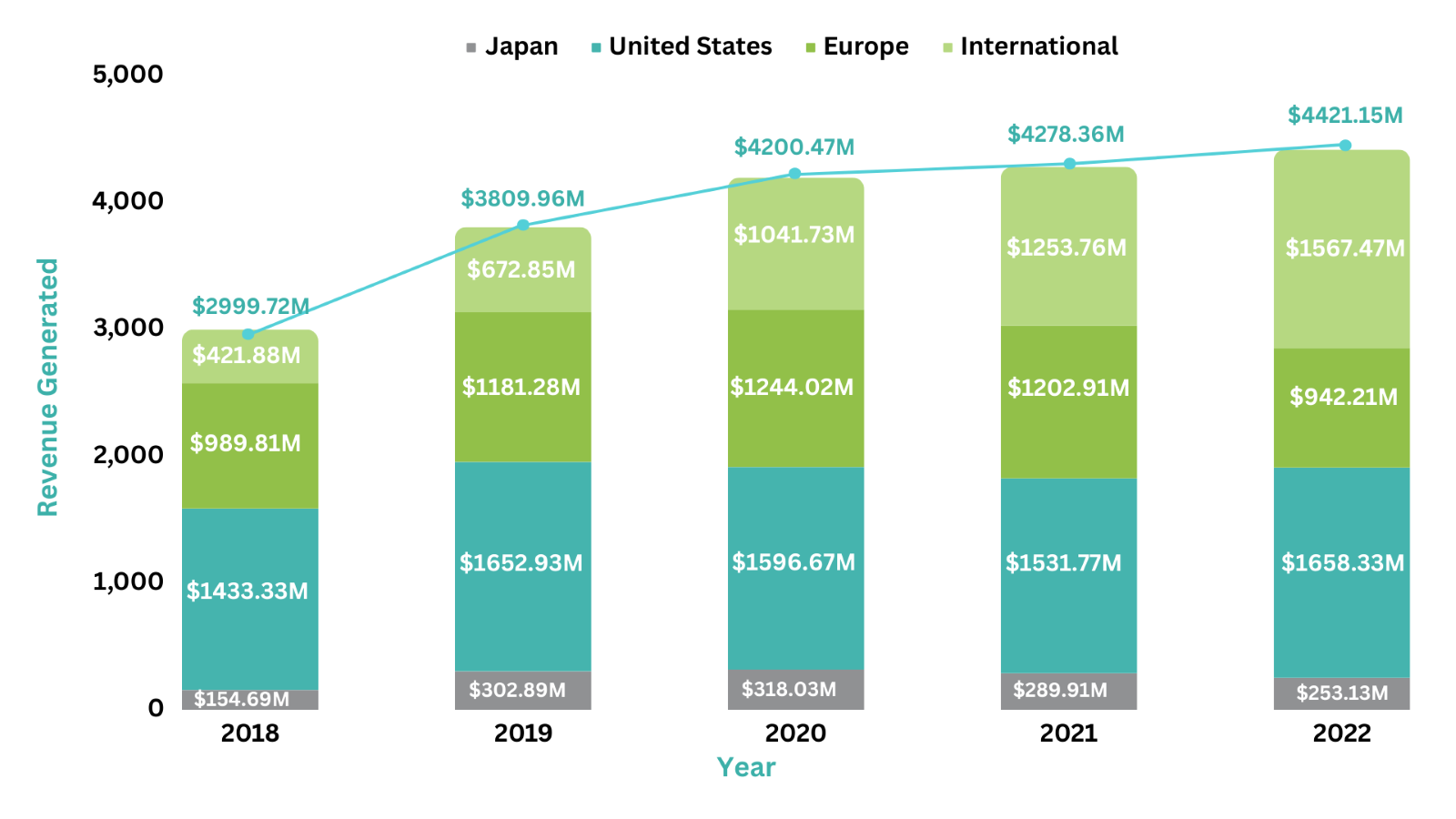
Revenue Analysis8
The sales of Genentech’s asset, Perjeta, have substantially grown to add a huge amount of profit to the company’s overall revenue. In 2022, Perjeta generated a total of $4,421.15M in sales, representing a 3.3% increase from 2021. Over 5 years, the highest percentage change in the product’s overall revenue was seen in the year 2019, with a 27% increase in sales as compared to the year 2018. This upsurge in the product’s sales was attributed to the increased use among patients with HER2-positive metastatic breast cancer. Moreover, in 2022, the overall sales of Perjeta were contributed by $1,567.47 from its sales in United States, $942.21M from its sales in Europe, $253.13M from its sales in Japan, and $1,658M from the International Markets.
The following graph illustrates the revenue analysis for the last five years' sales of Perjeta.
Approved Indications2, 3
Perjeta, in combination with Herceptin (trastuzumab) and chemotherapy (docetaxel in the EU), is indicated:
-
As a neoadjuvant treatment for HER2+, locally advanced, inflammatory, or early-stage breast cancer. It is used as part of a complete treatment regimen for early breast cancer (US and EU)
-
As an adjuvant therapy for the treatment of HER2+ early breast cancer that has a high chance of resurfacing (US)
-
For the treatment of adult patients with HER2+ metastatic or locally recurrent unresectable breast cancer and are not treated with prior anti-HER2 therapy or chemotherapy (EU)
Clinical Trials Analysis10
Clinical trial analysis is vital for advancing medical science, improving patient care, and making informed decisions about the safety and efficacy of new treatments. It underpins the foundation of evidence-based medicine and regulatory approval processes, leading to better healthcare outcomes and the development of innovative therapies.
The following dashboard illustrates the clinical trials associated with Perjeta.
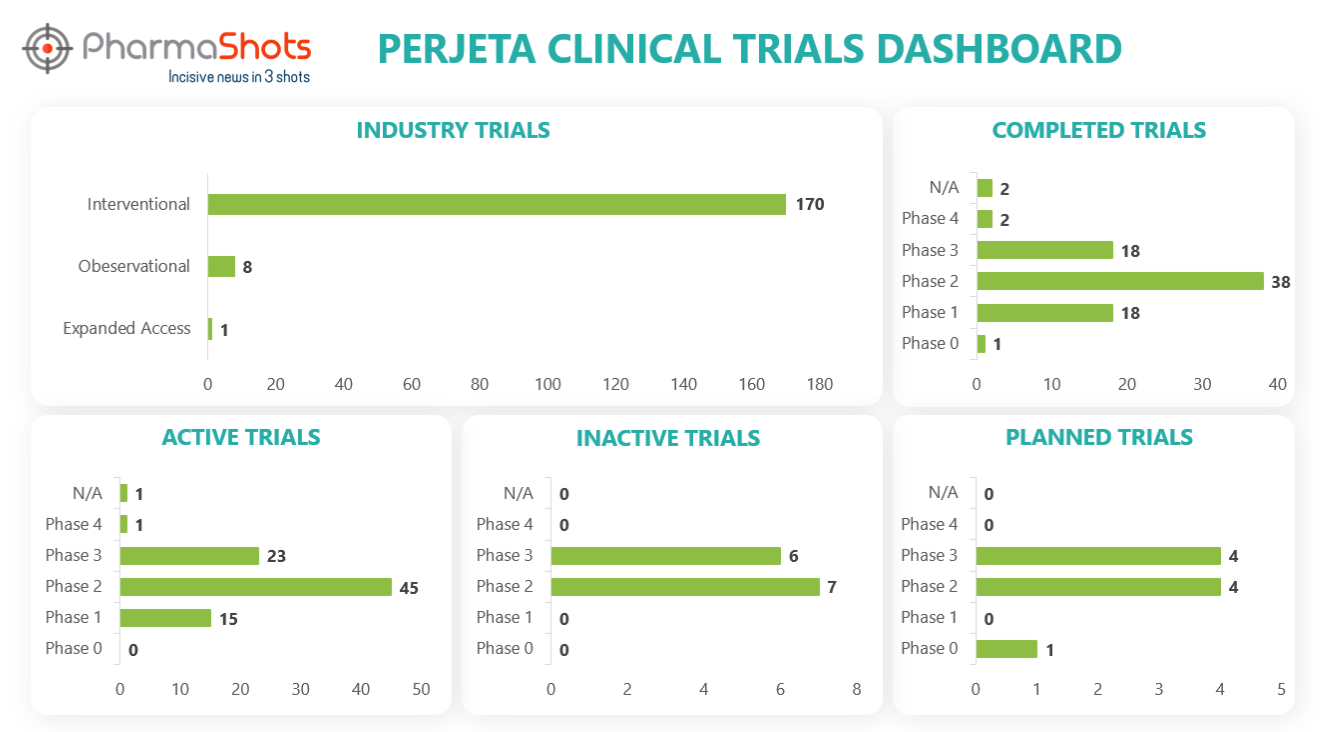
*Active trials include Recruiting; Active, Not Recruiting; Enrolling by Invitation, and suspended
*Inactive trials include Terminated; Withdrawn; Unknown Status
*Planned trials include Not, yet recruiting
Perjeta Trials Representation (Country-wise)10
Below graphs depict ongoing trials investigating Perjeta.
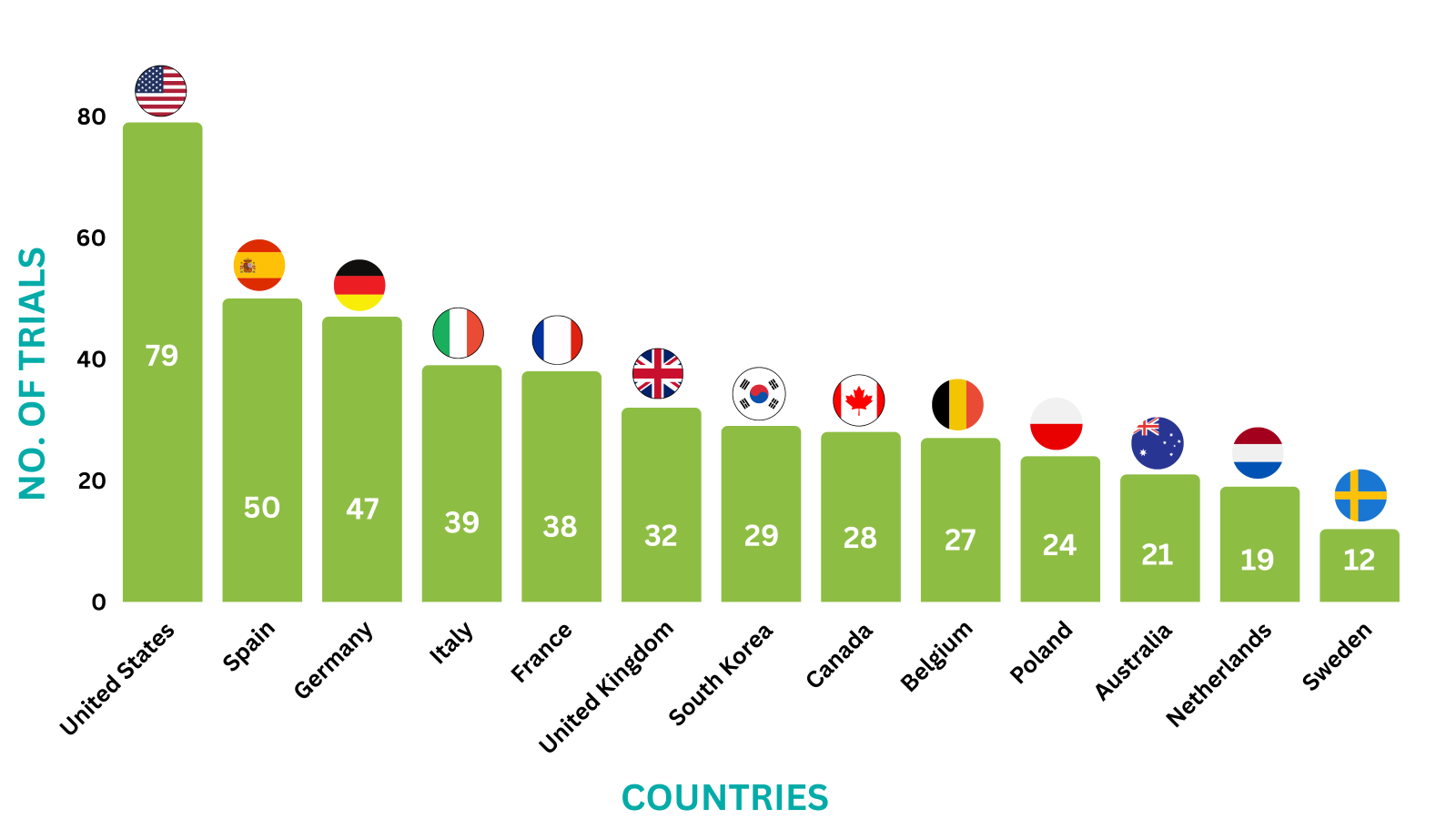
*The data represents trials till Jan 17, 2024
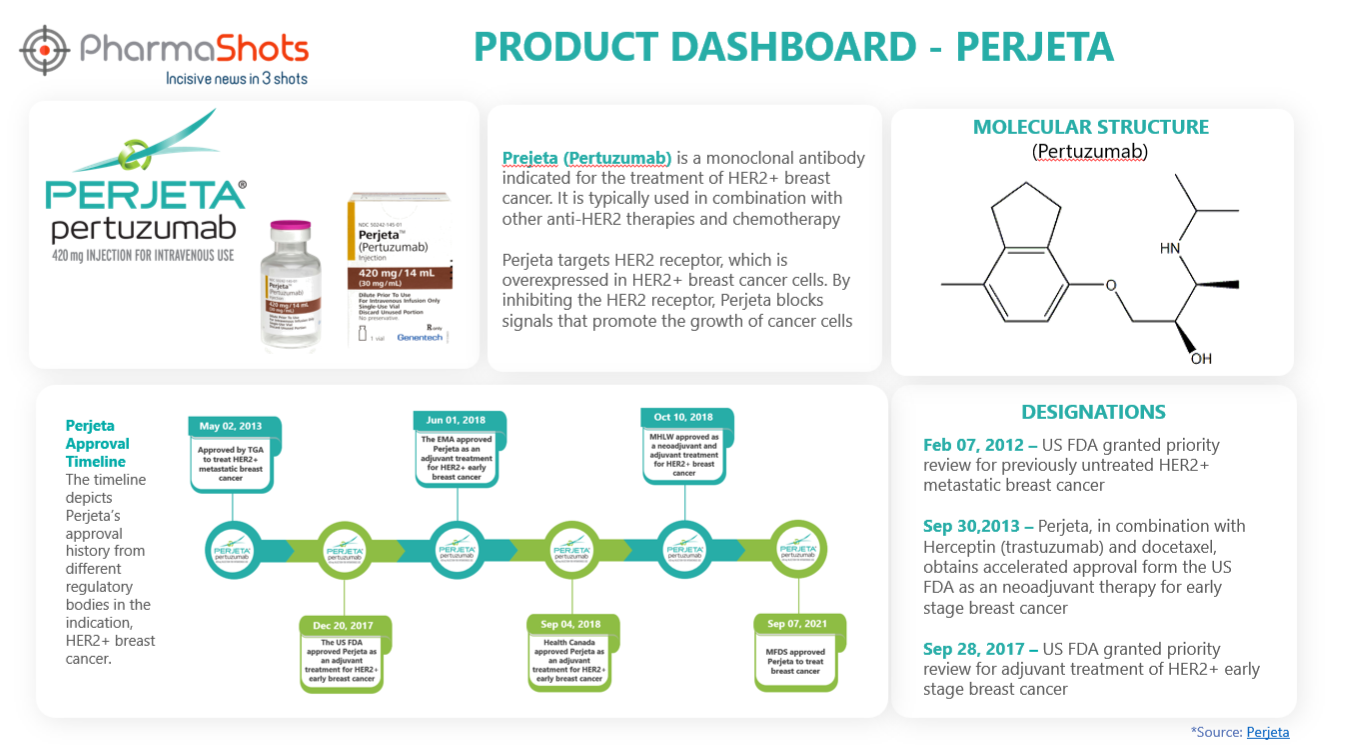
Product Dashboard
PharmaShots presents an illustrative infographic, highlighting essential metrics and pertinent information about Perjeta.
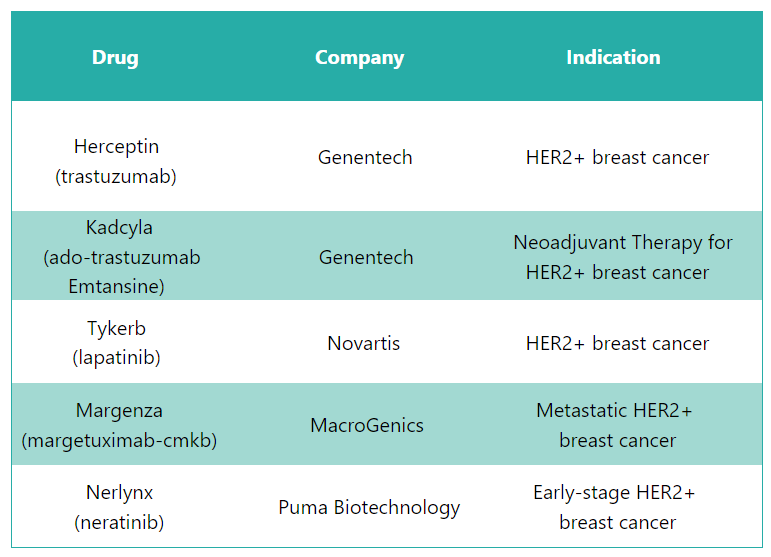
Perjeta Alternative Drugs1, 12
In response to Perjeta, several alternative drugs are available in the market to treat HER2- Positive Breast Cancer. Some of the substitute drugs for Perjeta include:
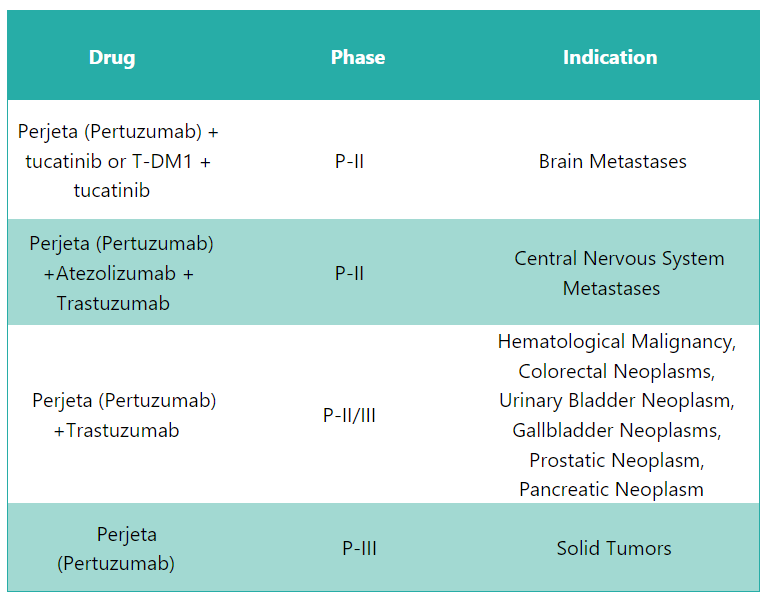
Perjeta Pipeline Analysis10
PharmaShots presents an extensive analysis of Perjeta’s pipeline, including the ongoing P-II and P-III studies for various indications. The table below depicts an overview of these studies.
Perjeta SWOT Analysis
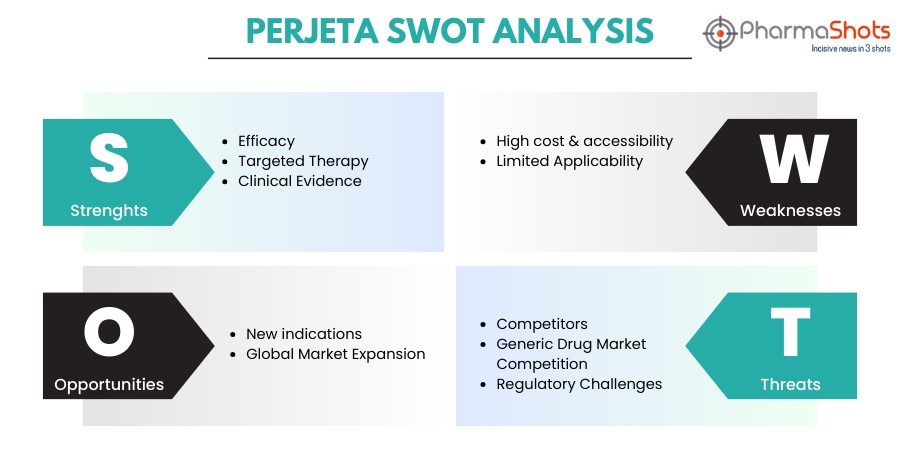
Strengths:
-
Efficacy: Patients with HER2-positive breast cancer have better results when Perjeta is used in combination with other HER2-targeted treatments including chemotherapy
-
Targeted Therapy: Perjeta is a targeted medication that minimizes harm to healthy cells and may lessen adverse effects by selectively targeting HER2-positive cancer cells
-
Clinical Evidence: Clinical trials and research provide credibility to Perjeta's effectiveness and offer a solid scientific basis for its use
Weaknesses
-
High Cost & Accessibility: As targeted medicines, like Perjeta, can be costly, some patients or healthcare providers may not be able to afford them
-
Limited Applicability: Perjeta's usage is limited to a subgroup of patients with breast cancer as it is only indicated for HER2-positive breast cancer
Opportunities
-
New Indications: There might be chances to investigate Perjeta's effectiveness in combination with other treatment modalities or in other HER2-positive malignancies
-
Global Market Expansion: There are prospects to enhance Perjeta's accessibility in international markets, hence broadening its patient base
Threats
-
Competitors: Since there is competition in the pharmaceutical sector, Perjeta's market share may be threatened by new treatments that develop
-
Generic Drug Market Competition: With age, Perjeta's cost and exclusivity on the market may be impacted by generic competition
-
Regulatory Challenges: The market expansion of Perjeta may face obstacles from modifications in regulatory standards or extensions in the approval of new indications.
Patient Stories13
Patients' stories are the key resources as they provide a holistic perspective on the impact of medications on individuals' lives, improve healthcare decision-making, and contribute to the overall understanding of the drug's efficacy and safety in real-world settings. We have summarized some of the patients’ stories for Perjeta:
-
KATE’S STORY: At the time of diagnosis, Kate found that her breast cancer had spread to the spine & while she received infusion treatment every 21 days, her body showed no evidence of cancer. Her oncologist reports that survival in HER2+ breast cancer improved & progression of the disease slowed down after taking trastuzumab (Herceptin) & pertuzumab (Perjeta) to Kate
-
LESLIE’S STORY: Leslie shares that in Sep 2014, she was at work & her right leg was shaking. When she visited the doctor she came to know that she had a benign tumor b/w the two hemispheres of her brain. After the surgery she found out that her breast cancer also spread to her right lung & spine then she started taking Taxotere, Herceptin, Perjeta, and Zometa. Currently, she is NED & enjoys doing things like yoga & running
KOL* Reviews7, 9
-
Dr. Karen Gelmon, Professor of Medicine, University of British Columbia says, "The addition of Perjeta to early treatment can lead to longer term survival without adding toxicity, and we already have good experience using Perjeta in other settings; this is great news for the Canadian breast cancer community."
-
Sandra Horning, CMO of Global Product Development says, “Today’s approval of Perjeta means people with HER2-positive early breast cancer at high risk of recurrence have a new, clinically meaningful treatment option to reduce the chances of their disease returning.”
-
MJ DeCoteau, Executive Director of Rethink Breast Cancer says, "This approval recognizes the importance of better, smarter treatment pathways that offer the best long-term results for people with breast cancer.”
-
Vaughan Gething, Minister of Economy, Wales Cancer Network says, “I’m pleased that Perjeta (pertuzumab) will now be routinely available on the Welsh NHS for people with advanced breast cancer. The Welsh Government’s flagship £80m New Treatment Fund is making more medicines available quicker than ever before. On average, new medicines are now being made available in only 10 days following recommendation. This means people with life-threatening conditions are getting much faster access to the latest medicines when they need them.”
*Key Opinion Leaders (KOLs) are crucial when it comes to the launch and assessment of pharmaceutical products. At Octavus, we recognize the importance of KOLs in the industry, which is why our proficient team dedicatedly tracks their activities and provides valuable insights to the pharma fraternity.
We understand that KOL tracking and selection can be overwhelming and time-consuming. That's why we offer extensive KOL tracking services to help our clients stay ahead of the curve. Our team of experts can provide you with the latest information on KOL activities, including their opinions, publications, and affiliations.
Interested in learning more about our KOL tracking services? Don't hesitate to reach out to us at bd@octavusconsulting.com or connect@pharmashots.com. We would be more than happy to provide you with more information and discuss how our services may benefit your business.
Octavus is a dedicated consulting company that offers a one-stop market solution to life science enterprises, biopharma, MedTech, diagnostic centers, digital health companies, animal healthcare, and start-ups.
References:
-
Drugs.com
-
Perjeta
-
EMA
-
TGA
-
Chugai Pharma
-
FDA
-
Newswire
-
Roche Sec filing
-
Gene.com
-
ClinicalTrials.gov
-
Genentech (Roche) PR
-
BreastCancer.org
-
Cleveland Clinic
Related Post: Top Performing Drug – Xtandi (December Edition)
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














